Limb Lengthening.us
Dror Paley, MD, FRCSC
Dror Paley, MD, FRCSC
| ORTHOPEDIC EDUCATIONAL SITE BY THE MOST EXPERIENCED LIMB LENGTHENING SURGEON IN THE WORLD |
By:
Dr. Dror Paley,
Director Paley Advanced Limb Lengthening Institute,
West Palm Beach, Florida.
For further inquiries contact Caroline Eaton ceaton@lengthening.us (561-307-8703)
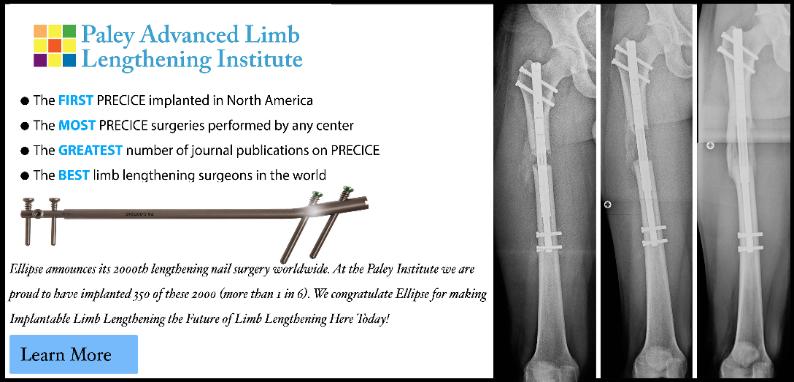
In August 2011, a new implantable lengthening device, the PRECICE, was approved by the FDA. It was developed by Ellipse
Technologies, out of California in conjunction with a team of orthopedic surgeon consultants, myself included. Ellipse used an
internal lengthening mechanism that they had developed for use in the spine. The major advance of this device is that it has
complete rate control and can even go reverse (shortening). Inside the lengthening nail there is magnet, which is connected to a
gearbox which in turn is connected to a screw shaft. Rotating the magnet, rotates the screw shaft and lengthens or shortens
the telescopic nail. To rotate the internal magnet there is an external actuator that is held by hand and applied to the limb. The
actuator has two magnets that are rotated by a motorized system while they are held against the leg at the level of the internal
magnet in the nail. It takes hundreds of revolutions of the external magnet to effect a 1 mm change in length of the nail. The
actuator lengthens the nail if it is facing one way and shortens it if it is facing the other way. It takes 7 minutes to achieve 1mm.
The nail is designed to be able to lengthen against a force of 80kg (176 lbs). The forces that need to be resisted inside the limb
have been reported to be up to 50 kg (110 lbs). Therefore this nail is more than strong enough to lengthen the limb. Although
each nail is for one time use, the actuator can be used for many patients. At present the FDA approved the use of the actuator
only for the physicians office. This means that the patient must come in to the office daily to have the lengthening performed,
including on weekends and holidays. The Precice can lengthen up to 6.5cms, although this amount may increase in future
models. Our orthopedic technologist performs the lengthening for each patient daily.
Dr. Dror Paley,
Director Paley Advanced Limb Lengthening Institute,
West Palm Beach, Florida.
For further inquiries contact Caroline Eaton ceaton@lengthening.us (561-307-8703)
In August 2011, a new implantable lengthening device, the PRECICE, was approved by the FDA. It was developed by Ellipse
Technologies, out of California in conjunction with a team of orthopedic surgeon consultants, myself included. Ellipse used an
internal lengthening mechanism that they had developed for use in the spine. The major advance of this device is that it has
complete rate control and can even go reverse (shortening). Inside the lengthening nail there is magnet, which is connected to a
gearbox which in turn is connected to a screw shaft. Rotating the magnet, rotates the screw shaft and lengthens or shortens
the telescopic nail. To rotate the internal magnet there is an external actuator that is held by hand and applied to the limb. The
actuator has two magnets that are rotated by a motorized system while they are held against the leg at the level of the internal
magnet in the nail. It takes hundreds of revolutions of the external magnet to effect a 1 mm change in length of the nail. The
actuator lengthens the nail if it is facing one way and shortens it if it is facing the other way. It takes 7 minutes to achieve 1mm.
The nail is designed to be able to lengthen against a force of 80kg (176 lbs). The forces that need to be resisted inside the limb
have been reported to be up to 50 kg (110 lbs). Therefore this nail is more than strong enough to lengthen the limb. Although
each nail is for one time use, the actuator can be used for many patients. At present the FDA approved the use of the actuator
only for the physicians office. This means that the patient must come in to the office daily to have the lengthening performed,
including on weekends and holidays. The Precice can lengthen up to 6.5cms, although this amount may increase in future
models. Our orthopedic technologist performs the lengthening for each patient daily.
This UNIQUE fellowship is designed for orthopedic surgeons
interested in developing an expertise in pediatric and adult limb
reconstruction surgery of the lower and upper extremities.
For more information Click Here
interested in developing an expertise in pediatric and adult limb
reconstruction surgery of the lower and upper extremities.
For more information Click Here
If there is any problem he alerts the clinical team, and the patient is seen by a physician assistant or doctor the same day. Since
patients undergo daily physical therapy (PT) sessions at the Paley Institute, we coordinate the lengthening session with the
physical therapy schedule. Patients have an x-ray every week to monitor the lengthening. The x-rays are measured to confirm
that the amount of lengthening that the actuator did has in fact been achieved. After the x-ray they are seen by one of our
doctors or PA’s.
The Precice heralds in a new era for limb lengthening. We now finally have a device that can be implanted with minimal incision
surgery and which can perform lengthening by a remotely controlled mechanism without rate control problems. The safety factor
with this device is excellent since it can be lengthened at any rate and can even be reversed to shorten the limb. Rate control
should eliminate most of the complications we saw with the ISKD. At present we are the only center in the US to implant this
device but we expect other centers to start using it.
Despite the ease of insertion and use, the limb lengthening process remains the same and the risks associated with limb
lengthening remain unchanged. For these reasons it is still essential that a surgeon experienced in limb lengthening and in the
treatment of lengthening complications be he one performing the procedure and following the patient. (see complications section
below)
Recovery from Implantable Limb Lengthening
The typical recovery from femoral or tibial lengthening is as follows:
1) surgery and hospitalization: 3-4 days
2) distraction phase; daily lengthening one mm per day; walking with crutches or walker; 50% weight bearing (WB) allowed
on the lengthened leg and full WB on the untreated leg; daily physical therapy (PT); patient allowed to shower, bathe and go in
swimming pool; patient allowed to drive 2 weeks after surgery (for patients over age 18)
3) consolidation phase until full WB permitted = 1 month in most but can be longer. The end of this phase is when the
bone on the x-ray appears to bridge the lengthening gap at least on one side. WB is progressed to full WB.
4) Rehabilitation phase: full WB without crutches. Regaining of muscle strength and joint range of motion to normal.
Usually 1-3 months.
5) Return to sports usually by 4-6 months after surgery.
Removal of Implant:
The implantable lengthening device should be removed. Although it is made of inert metal (either titanium or stainless steel),
there are also other materials including rare earth magnets, etc. The moving parts also can lead to wear and even corrosion. For
these reasons it is preferable to remove the device. The device can usually be removed as early as one year after surgery. There
is no urgency in the timing of removal but it should be done. The removal is an outpatient procedure. It can be deferred for
more than one year.
Historical perspective on implantable limb lengthening devices:
I have been performing Limb Lengthening Surgery since 1986. The two main indications for such surgery are limb length
equalization for limb length discrepancy (LLD) and stature lengthening for short stature. Since 1986 I have performed 13,000
limb lengthening surgeries. This is probably more than any other surgeon worldwide.
I started with the Ilizarov method for lengthening in 1987 and soon after switched to the lengthening over nail method I had
developed in 1990. This shortened the external fixation treatment time.
I sought a fully implantable lengthening solution. When the Alibizzia nail, developed by Guichet became available I worked with
the French company that made the nail to develop a tibial lengthening Albizzia. I started using both the femoral and tibial
Albizzia in 1996. The severe pain experienced by patients from the 15 rotation of the thigh through the break in the bone, as
well as several implant failures lead me to stop using this non-FDA approved device. In 2001, when the ISKD, was approved by
the FDA and marketed by Orthofix became available, I was the second surgeon to implant this device. I thought that this was
going to be the panacea for lengthening. I have since performed over 350 ISKD implantable limb lengthenings, more than
anyone in the world. The surgery was minimally invasive with few scars. The problem was rate control. The ISKD lengthening is
dependent on movement. Therefore it can lengthen too quickly, too slowly or at the desired rate of 1mm per day. Over 50% of
cases lengthened too quickly, 20% too slowly and only 30% at the desired rate. I was a consultant for Orthofix and advised
them since 2001 that they need to redesign the mechanism to achieve rate control. Lack of rate control lead to most of the
complications, such as muscle contractures, nerve injury, poor bone formation, etc. Furthermore there were many malfunctions
of the mechanism, which for unexplained reasons would fail to lengthen in the middle of the distraction phase. This lead to
increased numbers of procedures to treat complications. For stature patients this also meant increased costs. I learned to work
with the ISKD to minimize complications and became an expert at the treatment and prevention of these complications. My final
results due to my diligence were excellent in almost every patient. The ISKD was the only FDA approved device and was the
best implantable lengthening device that we had in the USA. The ISKD, the Albizzia are all what I call first generation lengthening
nails. They all suffer from significant mechanical and other problems.
On December 1, 2011, I implanted the first 3 Precice nails. I can attest to the perfect rate control in these three cases and the
complete lack of pain compared to the ISKD and Albizzia. While the procedure for implantation was the same with few and very
short incisions (minimal scars) the postoperative course thus far has been much more comfortable for the patients. I think this
difference is due to two factors: rate of lengthening control and no rotatory movement through the osteotomy site.
Here are links to the first bilateral PRECICE femoral lengthening in the news:
NEWS OK edmond-woman-takes-steps-to-lengthen-legs
South Florida Hospital News "Dror Paley is First Surgeon in US to Perform Limb Lengthening with New PRECICE Remote
Control Device"
Unexpected problems, complications and costs:
No one wants unexpected problems, complications and costs. For these reasons I am very conservative regarding many aspects
of the limb lengthening process. I try and anticipate problems and prevent complications. Many complications lead to additional
surgery and therefore to additional costs. The following is a list of the more common potential complications:
Premature consolidation: in this complication the patient bone bridges the gap and prevents further lengthening. Premature
consolidation (PC) can occur with any method if the patient is a very rapid bone healer. The patient in these cases is able to
make bone faster than the speed at which the bone is being lengthened. The only way to prevent this is to speed up the
lengthening intentionally for a week or two. The Precice nail with its rate control allows the surgeon to do this. If premature
consolidation does occur it requires an outpatient small surgery to rebreak the bone through a tiny incision.
With the ISKD and Albizzia premature consolidation was a well recognized complication due to the lack of control of rate of
lengthening. Since lengthening in both of these devices occurred by movement through the osteotomy site and since movement
through the osteotomy site can cause pain and muscle spasm, the patients muscles sometimes would prevent the movement
and therefore the lengthening from occurring. In other cases both the ISKD and the Albizzia have had broken mechanisms that
fail to lengthen during the distraction phase leading to PC. The treatment in these cases was to not only rebreak the bone but
also to change the device to a new device.
Delayed or failure of consolidation: slow or failed bone healing can occur with any lengthening surgery. This complication can
usually be prevented by making drill holes at the level of the planned osteotomy before reaming the bone. This is a technique I
introduced in 1990 with the lengthening over nail method. Stable fixation is also important so the choice of nail length and
diameter are important as well as the level of the osteotomy. Even the type of osteotomy affects the rate of bone healing.
Cutting the bone with multiple drill holes and an osteotomy is the most minimal invasive way while using an intramedullary saw
or performing an open osteotomy have higher failure rates. All of these are surgeon controlled parameters and are based on
surgeon knowledge and experience. Choosing the wrong level or method of osteotomy or the wrong diameter or length of
implant can significantly impact the result. Perhaps the most important parameter however is the rate of distraction.
Lengthening too quickly can lead to delay or complete or partial failure of bone formation.
Too rapid distraction is the most common cause of poor bone formation with the ISKD. This is not a problem with the Precice
since it has complete rate control. Poor bone healing can be recognized during the lengthening process. Once it is recognized
the rate of distraction should be slowed. Slowing the distraction is difficult with the ISKD. It requires the patient to stop physical
therapy, get into bed and decrease mobility and wear a brace from the hip to the ankle. With the Precise the lengthening can be
reduced to any level or even stopped. If despite these changes the bone healing remains poor, the lengthening can be reversed
until better bone formation is seen. The bone can then be relengthened. This can only be done with the Precise. Going reverse is
not possible with the ISKD or Albizzia. This is a huge advantage that was only possible before with external fixation.
Delay or failure of bone formation can delay weightbearing and increase the period of disability and recovery. Furthermore it can
lead to the need for surgery to get the bone to heal. Such surgery requires a bone graft and is not a small operation and can be
quite costly. Therefore having a device like the Precice that can prevent or treat the problem is a major advance.
Nerve injury: nerve injury can occur with any lengthening surgery but is usually uncommon if the rate of distraction does not
exceed 1mm per day and if the amount of lengthening is restricted. Rate control is the most important factor to prevent nerve
damage. Recognition of nerve symptoms is important. The lengthening should be stopped or slowed in such cases. If any motor
symptoms (weakness or paralysis of muscles) occurs a nerve decompression should be done as soon as possible. This is a
small outpatient surgery. In most cases it is the peroneal nerve that gets into trouble. It is important that the surgeon know
how to decompress this nerve to prevent foot drop. Delay in decompression can lead to permanent foot drop.
The ISKD too rapid distraction has lead to nerve complications in some patients. For this reason I will not lengthen more than
5cms with the ISKD. With the Precice and complete rate control, nerve injury should be much less common.
Muscle contractures: muscles normally get tight with lengthening. A muscle contracture occurs when a muscle gets tight
enough to prevent a joint from moving through its entire range of motion. To prevent muscle contractures physical therapy
(PT) is essential. The patient should do daily stretches of the muscles and joints at risk. E.g. knee joint and quadriceps muscles
for femur lengthening and ankle joint and Achilles tendon for tibial lengthening. In addition to formal PT the patient should do
their own stretches at home several times per day. PT is essential to the lengthening process. Too rapid distraction with the
ISKD made PT even more difficult. We frequently had to suspend PT to slow the distraction. We also had to fight muscle spasm
due to the constant bone movement with the ISKD. For this reason we started using Botox to prevent spasm with ISKD. It is
usually not necessary if the rate of distraction is controllable. Once again the controlled rate of lengthening with the Precice
makes the risk of muscle contractures and muscle spasm less. I do not routinely use Botox with the Precice which is another
cost savings. The Precise does not obviate the need for PT. Maintaining range of motion and preventing contractures during
lengthening decreases the rehabilitation time to return to normal function after the lengthening is finished. A fixed contracture
of the knee or ankle can lead to disability and the need for more prolonged PT and the expenses associated. If despite additional
PT the contracture does not resolve additional surgery to lengthen muscles, tendons and fascia may be required. I try and
anticipate this and prophylactically lengthen certain soft tissue structures to prevent contractures.
Fibular complications: with tibial lengthening the fibula has to be lengthened too. The implantable lengthening device only
lengthens and fixes the tibia. The fibula has to be fixed to the tibia so that it lengthens together with it. If the fibula is not fixed
or not fixed adequately it will not lengthen as much as the tibia and will lead to severe consequences including subluxation and
arthritis of the ankle and flexion contracture of the knee. The method of fixation is critical. Many surgeons only fix the lower end
of the fibula to the tibia. This can lead the fibula to prematurely consolidate and to pull down and dislocate from the tibia at its
upper end. It is important to fix the fibula at both ends. With external fixation the fibula can be fixed with the wires of an
external fixator. With implantable lengthening the fibula must be fixed with screws to the tibia; one screw at the upper end and
one at the lower end. The angle, level, position, diameter, and type of screw are all important. E.g. a common mistake is to put
the screw in horizontally between the two bones. This is not strong enough to prevent the fibula from pulling away from the
tibia at the ankle. This is very subtle and even a few millimeters of difference in length of the fibula at the ankle lead to short
term and/or long term consequences for the patient. Removing a segment of the fibula to prevent the fibula from not
separating is another common method that should be abandoned. It leads to a nonunion of the fibula which can lead to a stress
fracture at a later date in the tibia. Furthermore it usually does not prevent the fibula from pulling away from the tibia. Therefore
fibular complications have nothing to do with the type of implantable lengthening device but rather with the method the surgeon
chooses to fixate the fibula to the tibia and the method of cutting the fibula bone.
Surgeon’s Note:
Although limb lengthening has many potential complications, the biggest safeguard for a patient is the surgeons
experience not only in the performance of limb lengthening surgery, but also in the prevention and treatment of
complications. The larger the surgeons experience the safer is the surgery for the patient. As the most experienced
limb lengthening surgeon worldwide, I pride myself in being able to offer the safest, most reliable and reproducible
limb lengthening surgery in the world.
Dror Paley, MD
patients undergo daily physical therapy (PT) sessions at the Paley Institute, we coordinate the lengthening session with the
physical therapy schedule. Patients have an x-ray every week to monitor the lengthening. The x-rays are measured to confirm
that the amount of lengthening that the actuator did has in fact been achieved. After the x-ray they are seen by one of our
doctors or PA’s.
The Precice heralds in a new era for limb lengthening. We now finally have a device that can be implanted with minimal incision
surgery and which can perform lengthening by a remotely controlled mechanism without rate control problems. The safety factor
with this device is excellent since it can be lengthened at any rate and can even be reversed to shorten the limb. Rate control
should eliminate most of the complications we saw with the ISKD. At present we are the only center in the US to implant this
device but we expect other centers to start using it.
Despite the ease of insertion and use, the limb lengthening process remains the same and the risks associated with limb
lengthening remain unchanged. For these reasons it is still essential that a surgeon experienced in limb lengthening and in the
treatment of lengthening complications be he one performing the procedure and following the patient. (see complications section
below)
Recovery from Implantable Limb Lengthening
The typical recovery from femoral or tibial lengthening is as follows:
1) surgery and hospitalization: 3-4 days
2) distraction phase; daily lengthening one mm per day; walking with crutches or walker; 50% weight bearing (WB) allowed
on the lengthened leg and full WB on the untreated leg; daily physical therapy (PT); patient allowed to shower, bathe and go in
swimming pool; patient allowed to drive 2 weeks after surgery (for patients over age 18)
3) consolidation phase until full WB permitted = 1 month in most but can be longer. The end of this phase is when the
bone on the x-ray appears to bridge the lengthening gap at least on one side. WB is progressed to full WB.
4) Rehabilitation phase: full WB without crutches. Regaining of muscle strength and joint range of motion to normal.
Usually 1-3 months.
5) Return to sports usually by 4-6 months after surgery.
Removal of Implant:
The implantable lengthening device should be removed. Although it is made of inert metal (either titanium or stainless steel),
there are also other materials including rare earth magnets, etc. The moving parts also can lead to wear and even corrosion. For
these reasons it is preferable to remove the device. The device can usually be removed as early as one year after surgery. There
is no urgency in the timing of removal but it should be done. The removal is an outpatient procedure. It can be deferred for
more than one year.
Historical perspective on implantable limb lengthening devices:
I have been performing Limb Lengthening Surgery since 1986. The two main indications for such surgery are limb length
equalization for limb length discrepancy (LLD) and stature lengthening for short stature. Since 1986 I have performed 13,000
limb lengthening surgeries. This is probably more than any other surgeon worldwide.
I started with the Ilizarov method for lengthening in 1987 and soon after switched to the lengthening over nail method I had
developed in 1990. This shortened the external fixation treatment time.
I sought a fully implantable lengthening solution. When the Alibizzia nail, developed by Guichet became available I worked with
the French company that made the nail to develop a tibial lengthening Albizzia. I started using both the femoral and tibial
Albizzia in 1996. The severe pain experienced by patients from the 15 rotation of the thigh through the break in the bone, as
well as several implant failures lead me to stop using this non-FDA approved device. In 2001, when the ISKD, was approved by
the FDA and marketed by Orthofix became available, I was the second surgeon to implant this device. I thought that this was
going to be the panacea for lengthening. I have since performed over 350 ISKD implantable limb lengthenings, more than
anyone in the world. The surgery was minimally invasive with few scars. The problem was rate control. The ISKD lengthening is
dependent on movement. Therefore it can lengthen too quickly, too slowly or at the desired rate of 1mm per day. Over 50% of
cases lengthened too quickly, 20% too slowly and only 30% at the desired rate. I was a consultant for Orthofix and advised
them since 2001 that they need to redesign the mechanism to achieve rate control. Lack of rate control lead to most of the
complications, such as muscle contractures, nerve injury, poor bone formation, etc. Furthermore there were many malfunctions
of the mechanism, which for unexplained reasons would fail to lengthen in the middle of the distraction phase. This lead to
increased numbers of procedures to treat complications. For stature patients this also meant increased costs. I learned to work
with the ISKD to minimize complications and became an expert at the treatment and prevention of these complications. My final
results due to my diligence were excellent in almost every patient. The ISKD was the only FDA approved device and was the
best implantable lengthening device that we had in the USA. The ISKD, the Albizzia are all what I call first generation lengthening
nails. They all suffer from significant mechanical and other problems.
On December 1, 2011, I implanted the first 3 Precice nails. I can attest to the perfect rate control in these three cases and the
complete lack of pain compared to the ISKD and Albizzia. While the procedure for implantation was the same with few and very
short incisions (minimal scars) the postoperative course thus far has been much more comfortable for the patients. I think this
difference is due to two factors: rate of lengthening control and no rotatory movement through the osteotomy site.
Here are links to the first bilateral PRECICE femoral lengthening in the news:
NEWS OK edmond-woman-takes-steps-to-lengthen-legs
South Florida Hospital News "Dror Paley is First Surgeon in US to Perform Limb Lengthening with New PRECICE Remote
Control Device"
Unexpected problems, complications and costs:
No one wants unexpected problems, complications and costs. For these reasons I am very conservative regarding many aspects
of the limb lengthening process. I try and anticipate problems and prevent complications. Many complications lead to additional
surgery and therefore to additional costs. The following is a list of the more common potential complications:
Premature consolidation: in this complication the patient bone bridges the gap and prevents further lengthening. Premature
consolidation (PC) can occur with any method if the patient is a very rapid bone healer. The patient in these cases is able to
make bone faster than the speed at which the bone is being lengthened. The only way to prevent this is to speed up the
lengthening intentionally for a week or two. The Precice nail with its rate control allows the surgeon to do this. If premature
consolidation does occur it requires an outpatient small surgery to rebreak the bone through a tiny incision.
With the ISKD and Albizzia premature consolidation was a well recognized complication due to the lack of control of rate of
lengthening. Since lengthening in both of these devices occurred by movement through the osteotomy site and since movement
through the osteotomy site can cause pain and muscle spasm, the patients muscles sometimes would prevent the movement
and therefore the lengthening from occurring. In other cases both the ISKD and the Albizzia have had broken mechanisms that
fail to lengthen during the distraction phase leading to PC. The treatment in these cases was to not only rebreak the bone but
also to change the device to a new device.
Delayed or failure of consolidation: slow or failed bone healing can occur with any lengthening surgery. This complication can
usually be prevented by making drill holes at the level of the planned osteotomy before reaming the bone. This is a technique I
introduced in 1990 with the lengthening over nail method. Stable fixation is also important so the choice of nail length and
diameter are important as well as the level of the osteotomy. Even the type of osteotomy affects the rate of bone healing.
Cutting the bone with multiple drill holes and an osteotomy is the most minimal invasive way while using an intramedullary saw
or performing an open osteotomy have higher failure rates. All of these are surgeon controlled parameters and are based on
surgeon knowledge and experience. Choosing the wrong level or method of osteotomy or the wrong diameter or length of
implant can significantly impact the result. Perhaps the most important parameter however is the rate of distraction.
Lengthening too quickly can lead to delay or complete or partial failure of bone formation.
Too rapid distraction is the most common cause of poor bone formation with the ISKD. This is not a problem with the Precice
since it has complete rate control. Poor bone healing can be recognized during the lengthening process. Once it is recognized
the rate of distraction should be slowed. Slowing the distraction is difficult with the ISKD. It requires the patient to stop physical
therapy, get into bed and decrease mobility and wear a brace from the hip to the ankle. With the Precise the lengthening can be
reduced to any level or even stopped. If despite these changes the bone healing remains poor, the lengthening can be reversed
until better bone formation is seen. The bone can then be relengthened. This can only be done with the Precise. Going reverse is
not possible with the ISKD or Albizzia. This is a huge advantage that was only possible before with external fixation.
Delay or failure of bone formation can delay weightbearing and increase the period of disability and recovery. Furthermore it can
lead to the need for surgery to get the bone to heal. Such surgery requires a bone graft and is not a small operation and can be
quite costly. Therefore having a device like the Precice that can prevent or treat the problem is a major advance.
Nerve injury: nerve injury can occur with any lengthening surgery but is usually uncommon if the rate of distraction does not
exceed 1mm per day and if the amount of lengthening is restricted. Rate control is the most important factor to prevent nerve
damage. Recognition of nerve symptoms is important. The lengthening should be stopped or slowed in such cases. If any motor
symptoms (weakness or paralysis of muscles) occurs a nerve decompression should be done as soon as possible. This is a
small outpatient surgery. In most cases it is the peroneal nerve that gets into trouble. It is important that the surgeon know
how to decompress this nerve to prevent foot drop. Delay in decompression can lead to permanent foot drop.
The ISKD too rapid distraction has lead to nerve complications in some patients. For this reason I will not lengthen more than
5cms with the ISKD. With the Precice and complete rate control, nerve injury should be much less common.
Muscle contractures: muscles normally get tight with lengthening. A muscle contracture occurs when a muscle gets tight
enough to prevent a joint from moving through its entire range of motion. To prevent muscle contractures physical therapy
(PT) is essential. The patient should do daily stretches of the muscles and joints at risk. E.g. knee joint and quadriceps muscles
for femur lengthening and ankle joint and Achilles tendon for tibial lengthening. In addition to formal PT the patient should do
their own stretches at home several times per day. PT is essential to the lengthening process. Too rapid distraction with the
ISKD made PT even more difficult. We frequently had to suspend PT to slow the distraction. We also had to fight muscle spasm
due to the constant bone movement with the ISKD. For this reason we started using Botox to prevent spasm with ISKD. It is
usually not necessary if the rate of distraction is controllable. Once again the controlled rate of lengthening with the Precice
makes the risk of muscle contractures and muscle spasm less. I do not routinely use Botox with the Precice which is another
cost savings. The Precise does not obviate the need for PT. Maintaining range of motion and preventing contractures during
lengthening decreases the rehabilitation time to return to normal function after the lengthening is finished. A fixed contracture
of the knee or ankle can lead to disability and the need for more prolonged PT and the expenses associated. If despite additional
PT the contracture does not resolve additional surgery to lengthen muscles, tendons and fascia may be required. I try and
anticipate this and prophylactically lengthen certain soft tissue structures to prevent contractures.
Fibular complications: with tibial lengthening the fibula has to be lengthened too. The implantable lengthening device only
lengthens and fixes the tibia. The fibula has to be fixed to the tibia so that it lengthens together with it. If the fibula is not fixed
or not fixed adequately it will not lengthen as much as the tibia and will lead to severe consequences including subluxation and
arthritis of the ankle and flexion contracture of the knee. The method of fixation is critical. Many surgeons only fix the lower end
of the fibula to the tibia. This can lead the fibula to prematurely consolidate and to pull down and dislocate from the tibia at its
upper end. It is important to fix the fibula at both ends. With external fixation the fibula can be fixed with the wires of an
external fixator. With implantable lengthening the fibula must be fixed with screws to the tibia; one screw at the upper end and
one at the lower end. The angle, level, position, diameter, and type of screw are all important. E.g. a common mistake is to put
the screw in horizontally between the two bones. This is not strong enough to prevent the fibula from pulling away from the
tibia at the ankle. This is very subtle and even a few millimeters of difference in length of the fibula at the ankle lead to short
term and/or long term consequences for the patient. Removing a segment of the fibula to prevent the fibula from not
separating is another common method that should be abandoned. It leads to a nonunion of the fibula which can lead to a stress
fracture at a later date in the tibia. Furthermore it usually does not prevent the fibula from pulling away from the tibia. Therefore
fibular complications have nothing to do with the type of implantable lengthening device but rather with the method the surgeon
chooses to fixate the fibula to the tibia and the method of cutting the fibula bone.
Surgeon’s Note:
Although limb lengthening has many potential complications, the biggest safeguard for a patient is the surgeons
experience not only in the performance of limb lengthening surgery, but also in the prevention and treatment of
complications. The larger the surgeons experience the safer is the surgery for the patient. As the most experienced
limb lengthening surgeon worldwide, I pride myself in being able to offer the safest, most reliable and reproducible
limb lengthening surgery in the world.
Dror Paley, MD
Paley Institute Limb Reconstruction Fellowship Program

Limb Length Discrepancy (LLD)
Advances made by Dr. Paley in the past 27 years
Limb Lengthening: A New Breakthrough
Cosmetic Stature Lengthening & Frequently Asked Questions
(FAQ’s) CLICK HERE
Advances made by Dr. Paley in the past 27 years
Limb Lengthening: A New Breakthrough
Cosmetic Stature Lengthening & Frequently Asked Questions
(FAQ’s) CLICK HERE